COMPANY NEWS
Key points for the construction of clinical gene amplification (PCR) laboratory
A clinical gene amplification laboratory is also called a PCR laboratory. PCR is the abbreviation of polymerase chain reaction, which is a molecular biology technology used to amplify specific DNA fragments. It can be regarded as a special DNA replication outside the body. Through the DNA gene tracking system, the virus content in the patient can be quickly grasped, and its accuracy is as high as a nanometer level.
Because it can detect viruses that are difficult to detect with ordinary tests and has the advantages of high sensitivity, high specificity, fast speed, and low sample requirements, it is recognized by clinical medicine and has been widely used in clinical diagnosis of hospitals and poultry disease diagnosis in epidemic prevention and detection departments, such as: detection and diagnosis of AIDS, hepatitis B, cancer genes, DNA fingerprinting, individual identification, paternity testing, and forensic evidence testing, animal and plant quarantine, animal and derivative product testing, animal feed, cosmetics, food hygiene testing, genetically modified crops and genetically modified microorganisms testing, etc.
PCR experiments require a laboratory that can guarantee absolute safety, reasonable configuration, and very standardized operation. In this article, we will explain the construction points of the three aspects of the layout of the gene amplification laboratory, the design and pressure control of the air conditioning and ventilation system, and the prevention and control of pollution.
1. PCR laboratory layout
In principle, the PCR laboratory should have dedicated channels (corridors) and four separate working areas: reagent preparation area (reagent storage and preparation), specimen preparation area, PCR amplification area (amplification reaction mixture preparation and amplification), and product analysis area (amplification product analysis). Independent PCR laboratories should also have sample-receiving rooms, autoclaves, changing rooms, restrooms, sanitary rooms, etc.
To avoid cross-contamination, entry into each working area must strictly follow the principle of a single direction, that is, only from the reagent preparation area → specimen preparation area → PCR amplification area → product analysis area.
The transfer of reagents and samples between experimental areas should be carried out through transfer windows.
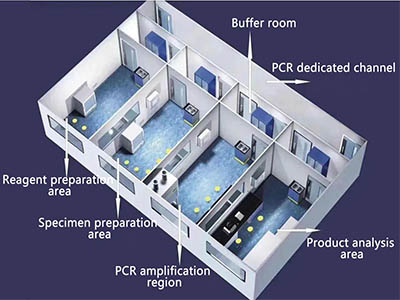
2. Laboratory air conditioning and ventilation system design and pressure control
To avoid the possibility of cross-contamination between experimental areas, PCR laboratories should adopt a full-supply and full-exhaust airflow organization. At the same time, the proportion of supply and exhaust should be strictly controlled to ensure the pressure requirements of each experimental area. The layout of the air supply and exhaust vents in the buffer zone and core work area of the laboratory using a mechanical ventilation system should comply with the principle of directional airflow, reduce vortices and airflow dead corners in the room, and the exhaust vents should be located in the area with the highest risk of indoor contamination, with a one-way layout and no obstacles. It is advisable to adopt the upper supply and lower exhaust method.
1. Reagent preparation area
The main operations in this experimental area are the preparation of storage reagents, the packaging of reagents, and the preparation of the main reaction mixture. Reagents and materials used for specimen preparation should be transported directly to this area and must not pass through other areas. Reagent raw materials must be stored in this area and prepared into the required storage reagents in this area.
The reagent preparation area is a positive pressure environment with a pressure of 5~10pa, which can protect the reagent preparation from external contamination.
2. Specimen preparation area
The main operations in this area are specimen preservation, nucleic acid (RNA, DNA) extraction, storage, and addition to amplification reaction tubes and cDNA synthesis when measuring RNA.
The specimen preparation area is a negative pressure environment with a pressure of -10pa, which can protect personnel from harmful aerosol infection, protect samples from external contamination, and protect samples from cross infection. In addition, since aerosol contamination may occur during operation, unnecessary movement in this area should be avoided.
3. PCR amplification area
The main operation in this area is DNA or cDNA amplification. In addition, the addition of prepared DNA templates and synthesized cDNA (from the specimen preparation area) and the preparation of the main reaction mixture (from the reagent preparation area) into a reaction mixture can also be carried out in this area.
The amplification area is a negative pressure environment with a pressure of -15pa. To avoid contamination caused by aerosols, unnecessary movement in this area should be minimized. Individual operations such as sample addition should be carried out in a clean bench.
4. Product analysis area
The main operation in this area is the determination of amplified fragments. This area is the main source of contamination of amplified products and should be a negative pressure environment with a pressure of -20pa to prevent the amplified products from spreading from this area to other areas.
![]()
III. Prevention and control of contamination
The core issue of PCR laboratory design is how to avoid contamination. In actual work, the following types of contamination are common: contamination of amplified products, contamination of natural genomic DNA, contamination of reagents, and contamination between specimens. Once contamination occurs, the experiment must be stopped until the source of contamination is found, the experimental results must be invalidated and the experiment must be repeated. Therefore, searching for the source of contamination around the laboratory after contamination occurs is not only time-consuming and tedious, but also wastes manpower and material resources. To avoid contamination, prevention should be the first priority, not elimination.
1. Strict division of work areas
(1) Each experimental area should be reasonably set up.
(2) Each experimental area should have obvious markings (such as eye-catching doorplates or different floor colors, etc.) to avoid confusion between equipment, items, reagents, etc. in different experimental areas.
2. Reasonable and necessary facilities and system settings
(1) Reasonable air conditioning and ventilation system settings, try to use a full-supply and full-exhaust air conditioning system.
(2) Strict air flow pressure control to ensure different pressure requirements in different experimental areas.
(3) The autoclave should be equipped with an internal exhaust autoclave, a water tank, an exhaust fan, etc.
(4) PCR dedicated channels (corridors), buffer rooms, and core work area roofs should be equipped with UV lamps that meet the requirements (power ≥ 1.5w/m³), and the irradiation intensity of the UV lamps should be monitored regularly.
(5) PCR dedicated channels (corridors) should be equipped with clean hand wash basins, emergency spray devices, and eye washers. The faucets should be induction-type, elbow-open or foot-operated, rather than manual switches.

3. Formulate standard operating procedures (SOP)
1) Technicians in gene amplification laboratories must undergo on-the-job training and can only engage in clinical gene amplification testing after passing the training.
(2) During the experimental operation, the operator must wear gloves and change them frequently. In addition, the use of disposable hats during operation is also an effective measure to prevent contamination.
(3) Cleaning work should be timely and correct. After the experimental work is completed, the area must be cleaned immediately. In addition to using conventional disinfectant liquid to wipe and disinfect the surface or disinfect it with ultraviolet light, some experimental equipment should also be sterilized by high pressure.
(4) Each high-pressure sterilization must use chemical methods to verify the disinfection effect and keep disinfection and verification records; according to the biosafety risk assessment, biological monitoring of the high-pressure sterilization effect can be carried out once a month or quarterly according to the operating requirements.
4. Strict management
(1) Strictly control the personnel entering and leaving the laboratory. Personnel not related to the experiment shall not enter and leave the laboratory at will, and unnecessary movement in the experimental area shall be minimized to reduce the possibility of cross-contamination.
(2) Use work clothes with obvious distinguishing marks (such as different colors) in each experimental area. When the staff leaves, they shall not take the work clothes in this area to other areas.
(3) The amplification product analysis area is the main source of amplification product contamination. Waste liquid cannot be dumped in the laboratory. It must be soaked and disinfected with disinfectant and then sent to a designated location for unified treatment; used disposable materials such as pipette tips should also be soaked and disinfected with disinfectant and then uniformly treated.
(4) The amplification product analysis area may use certain substances that may cause gene mutations or be toxic. Special attention should be paid to the safety protection of the experimenters.
(5) Archives should be established for major equipment (nucleic acid extractors, amplifiers, biosafety cabinets, etc.), including: the name, model, serial number or other unique identification of the equipment, manufacturer name, date of receipt and date of activation, status at the time of receipt (e.g., brand new, modified), current location, instrument instruction manual or copy, date and results of calibration and/or verification and date of next calibration and/or verification, details of maintenance performed to date and future maintenance plans, historical records of damage, failure, modification or repair, etc.
(6) All instruments and equipment should be clearly marked, including instrument and equipment management identification and status identification. If an instrument or equipment fails, it should be stopped from use immediately and a fault identification should be added. After the fault is repaired, the equipment must be calibrated/verified (validated) or tested, and can only be put into use again after meeting the requirements, and the corresponding records should be retained. At the same time, the impact of the above defects on the previous inspection work should be checked.
5. Complete laboratory equipment and instruments
Complete equipment and instruments are necessary to ensure experimental work. According to the different experimental contents of each laboratory, corresponding equipment and instruments should be equipped, such as nucleic acid extractors, amplifiers, biosafety cabinets, fume hoods, refrigerators, clean benches, centrifuges, water baths, autoclaves, pipettes, thermometers, etc.
