PRODUCT
pharmaceutical pass through decontamination VHP pass box Vaporized hydrogen peroxide pass box
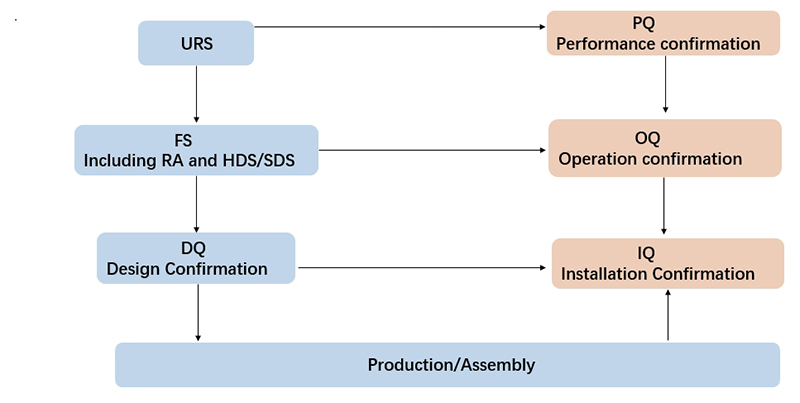
The sterilization time requirements of the VHP transfer window are as follows: empty load rapid sterilization function, with a sterilization time of ≤45 minutes; full load sterilization time of ≤60 minutes; and process temperature of ≤37℃. The validation document system complies with the requirements of cGMP and GAMP5. We can provide validation documents and services from URS, FS, DQ, FAT, SAT, IQ, OQ, and PQ.
VHP medicine pass box biological safety pharmaceutical clean room vhp throug
The VHP sterilization pass box is installed across clean levels, so it should have complete biological sealing. The panels, dashboards, signal lights, etc. on the unloading area side must be completely sealed to ensure the relative sealing of the high-purification level area relative to the low-purification level area and the outside world. (Comply with the following certifications: CE, LVD+MD, and national biosafety inspection report) and provide report certification documents.
•Product Principle
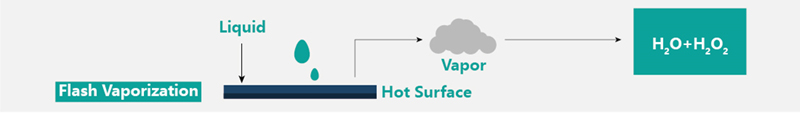
VHP technology refers to the low-temperature sterilization technology of vaporizing liquid hydrogen peroxide into hydrogen peroxide vapor and using vaporized hydrogen peroxide to sterilize the surface of objects.
VHP has a broad spectrum of bactericidal properties and can effectively kill all types of microorganisms such as bacteria, fungi, molds, viruses, bacterial spores, etc. It is currently found that the most difficult microorganisms to kill with VHP are thermophilic fat spores, so the biological indicator used for VHP sterilization validation is thermophilic fat spores.
• VHP sterilization is non-toxic and has no residue. Vaporized hydrogen peroxide can quickly kill microorganisms during the sterilization process. After sterilization, it can quickly degrade into H20 and 02, which is non-toxic and has no residue, and the residual concentration of hydrogen peroxide can be detected.
• The sterilization effect of VHP can be verified. A normal validation cycle includes parameter development, VHP distribution research, biological challenge test and exhaust degradation research. The vaporized hydrogen peroxide equipment has a complete GMP validation document system.
• VHP sterilization has good compatibility. The series of vaporized hydrogen peroxide sterilizers adopt a unique saturation control method to ensure that hydrogen peroxide does not liquefy or condense during the entire sterilization process, and the material compatibility is better.
The sterilization process is stable, with good diffusion, fast sterilization speed, no condensation, and no residue after sterilization.
You can choose a self-cleaning program, concentration program, etc. to meet different sterilization loading requirements. The sterilization time requirements of the VHP transfer window are empty load rapid sterilization function, sterilization time ≤45 minutes; full load sterilization time ≤60 minutes; process temperature ≤37℃.
Equipment body
The VHP transfer cabin adopts a stainless steel main structure, the inner cavity adopts 316L stainless steel, the frame, and outer surface adopt 304 stainless steel structure, the inner cavity adopts a rounded corner full welding design, and adopts a Ra≤0.6um polishing degree.
VHP generation unit
The dry VHP generator based on the flash evaporation principle adopts an integrated control method and adopts a unified control method with the VHP transfer window. The VHP generation concentration, cavity temperature, humidity, and saturation control are more stable.
Pneumatic sealing system.
The compressed air power system includes the control of the inflatable seal and the pneumatic valve. The inflatable seal and pneumatic valve control use a compressed air pipeline, including a pressure-reducing valve and a solenoid valve control. The other compressed air is controlled by an independent pressure-reducing valve and a solenoid valve for cavity saturation control.
Control system
The standard control system adopts PLC and HMI control mode, and adopts standard modular control board, which is stable and reliable. The control system design has been fully verified and applied in practice and is stable and reliable.
Purification and filtration system
The cavity air supply and exhaust are filtered by H14 high-efficiency filters. The process pipeline and cavity are both designed for purification. With the high-efficiency filtration system, Class A purification can be achieved in the cavity.
Electrical system
The design of the electrical cabinet meets the requirements of safety and protection regulations, the layout of electrical circuits is orderly and reasonable, and the strong and weak currents are clearly marked, which meets CE and EN standards.
EHS requirements
Full consideration has been given to personnel and product safety protection in the design. Strong electric safety protection is used to ensure that operators cannot come into contact with strong electricity. There are emergency stop buttons on both sides. High-temperature parts are clearly marked with high temperature. The cavity adopts electromagnetic interlocking devices to avoid contamination of products and clean rooms. Abnormal state sound and light alarm signals are used to prompt to avoid safety incidents.

Start: Before sterilization starts, the device automatically determines whether the sterilization conditions meet the sterilization start requirements, that is, temperature>15°C, 40%<RH%<60%;
Balance: The VHP generator starts to generate hydrogen peroxide vapor and circulates in the space until the preset concentration or saturation is reached;
Sterilization: The device starts to record the sterilization effect until the preset sterilization LOG value is reached;
Degradation: After the sterilization process ends, the device automatically enters the VHP degradation stage and starts to catalytically degrade the hydrogen peroxide vapor inside the device and the cavity until the set hydrogen peroxide concentration is reached
|
Conventional parts |
|
|
PLC |
SIEMENS S7-2000smart |
|
Operation panel - feed side touch screen |
MCGS |
|
Printer |
Wei Huang |
|
Motor |
EBM |
|
Concentration probe |
ATI high concentration probe/Vaisala three-in-one concentration probe |
|
Electrical components |
SCHNEIDER |
|
Pneumatic Components |
Air TAC |
|
Temperature and humidity probe |
Vaisala |
|
Differential pressure gauge |
DWYER |
|
HEPA |
AN |
|
VHP Generator |
Our factory |





