PRODUCT
High efficiency VHP decontamination pass box. Designed for pharmaceutical cleanrooms, BSL labs, and vaccine facilities.
The sterilization time requirements of the VHP transfer window are as follows: empty load rapid sterilization function, with a sterilization time of ≤45 minutes; full load sterilization time of ≤60 minutes; and process temperature of ≤37℃. The validation document system complies with the requirements of cGMP and GAMP5. We can provide validation documents and services from URS, FS, DQ, FAT, SAT, IQ, OQ, and PQ.
VHP pass box sterilizer for pharmaceutical clean room car-t
VHP has a broad spectrum of bactericidal properties and can effectively kill all types of microorganisms such as bacteria, fungi, molds, viruses, bacterial spores, etc. It is currently found that the most difficult microorganisms to kill with VHP are thermophilic fat spores, so the biological indicator used for VHP sterilization validation is thermophilic fat spores.
• VHP sterilization is non-toxic and has no residue. Vaporized hydrogen peroxide can quickly kill microorganisms during the sterilization process. After sterilization, it can quickly degrade into H20 and 02, which is non-toxic and has no residue, and the residual concentration of hydrogen peroxide can be detected.
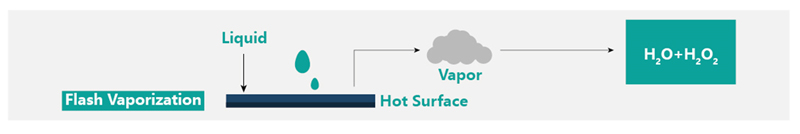
VHP pass box sterilizer Product Principle
• The sterilization effect of VHP can be verified. A normal validation cycle includes parameter development, VHP distribution research, biological challenge test and exhaust degradation research. The vaporized hydrogen peroxide equipment has a complete GMP validation document system.

• VHP sterilization has good compatibility. The vaporized hydrogen peroxide sterilizers adopt a unique saturation control method to ensure that hydrogen peroxide does not liquefy or condense during the entire sterilization process, and the material compatibility is better.
Equipment body
The VHP transfer cabin adopts a stainless steel main structure, the inner cavity adopts 316L stainless steel, the frame and outer surface adopt 304 stainless steel structure, the inner cavity adopts a rounded corner full welding design, and adopts a Ra≤0.6um polishing degree.
VHP generation unit
The dry VHP generator based on the flash evaporation principle adopts an integrated control method and adopts a unified control method with the VHP transfer window. The VHP generation concentration, cavity temperature, humidity, and saturation control are more stable.
Pneumatic sealing system.
The compressed air power system includes the control of the inflatable seal and the pneumatic valve. The inflatable seal and pneumatic valve control use a compressed air pipeline, including a pressure-reducing valve and a solenoid valve control. The other compressed air is controlled by an independent pressure-reducing valve and a solenoid valve for cavity saturation control.
|
Model |
Inner size (W*D*H) |
External size (W*D*H) |
Powe (kw) |
|
|
HPB-216 |
600*600*600mm |
1050*650*1800mm |
2.0 |
|
|
HPB-288 |
600*600*800mm |
1050*650*2000mm |
2.2 |
|
|
HPB-360 |
600*1000*600mm |
1050*1050*1800mm |
2.2 |
|
|
HPB-512 |
800*800*800mm |
1250*850*2000mm |
2.3 |
|
|
HPB-640 |
800*800*1000mm |
1250*850*2200mm |
2.5 |
|
|
HPB-800 |
800*1000*1000mm |
1250*1050*2200mm |
2.6 |
|
|
HPB-960 |
800*1000*1200mm |
1450*1250*2150mm |
2.8 |
|
|
HPB-1536 |
800*1200*1600mm |
1450*1250*2150mm |
3.0 |
|
|
HPB-1792 |
800*1400*1600mm |
1450*1450*2150mm |
3.2 |
|
|
HPB-2688 |
1200*1400*1600mm |
1850*1450*2150mm |
4.0 |
|
|
Note: The above are standard products, non-standard products can consult our sales staff, we can provide non-standard customized products according to customer requirements. |
||||
VHP pass box sterilizer Common material introduction
|
Item |
Power |
Compressed air |
Exhaust |
|
Requirements |
220v/50/Hz |
6-8Bar, RH%<30% clean compressed air |
Interface DN50 stainless steel pipe, Ø64mm flange quick clamp |





