PRODUCT
VHP pass box for pharmaceutical clean room Biological safety vhp throug
The VHP pass box, that is the hydrogen peroxide disinfection pass box, is mainly used for the sterilization of the outer packaging and outer surface of the material to avoid the contamination of the material from the low-level area to the high-level area. The hydrogen peroxide generator is used to sterilize the non-exposed surface in the pass box through the integrated vaporizing hydrogen peroxide, which belongs to the sterilization process at low temperatures and normal temperatures, which is more environmentally friendly, efficient, thorough and safe.
VHP pass box for pharmaceutical clean room Biological safety vhp throug
PRINCIPLE
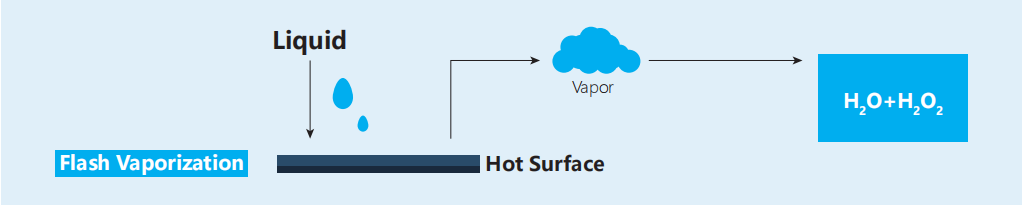
- VHP technology refers to the technology of vaporizing liquid hydrogen peroxide into hydrogen peroxide steam and using vaporized hydrogen peroxide to sterilize objects at low temperatures.
- VHP has a broad spectrum of bactericidal properties and can effectively kill all types of microorganisms such as bacteria, fungi, molds, viruses, and bacterial spores. Currently, it is found that the most difficult microorganism VHP to kill is thermophilic fat spores, so the biological indicator used for VHP sterilization verification is thermophilic fat spores.
- VHP sterilization is non-toxic and non-residual. Vaporized hydrogen peroxide can quickly kill microorganisms in the sterilization process, and can be rapidly degraded into water and oxygen after sterilization, which is non-toxic and non-residual, and the residual concentration of hydrogen peroxide can be detected.
- VHP sterilization effect can be verified. A normal verification cycle includes parameter development, VHP distribution study, biological challenge test and exhaust degradation study. HPB vaporizing hydrogen peroxide equipment has a complete GMP verification document system.
- VHP sterilization compatibility is good, the HPB series of vaporized hydrogen peroxide sterilizer adopts a unique saturation control method to ensure that the hydrogen peroxide does not liquefy or condense during the entire sterilization process, and the material compatibility is better.
VHP pass box for pharmaceutical clean room Biological safety vhp through
Laboratories: VHP Pass box is primarily used for the transfer of materials between sterile inspection laboratories, microbiology laboratories, positive control laboratories, and material transfer rooms, sterilization of material packaging and external surfaces.
Hospital: Material transfer in areas such as intensive care units, negative pressure isolation units, infectious units, pathology rooms and laboratory departments. And other material transfers that cannot be sterilized at high temperatures.
Equipment body
VHP pass box adopts stainless steel main structure, inner cavity adopts 316L stainless steel, frame and outer surface adopts 304 stainless steel structure, inner cavity adopts arc Angle full welding design, and adopts Ra≤0.6μm polishing degree.
Unit of VHP
Built-in flash principle dry VHP generator, integrated control mode, unified control mode with VHP pass box, VHP concentration, chamber temperature humidity and saturation control are more stable.
Pneumatic sealing system
The compressed air power system includes an inflatable seal and control of pneumatic valves. The charge seal and pneumatic valve control use a compressed air line, including pressure pressure-reducing valve and solenoid valve control. The other compressed air is controlled by an independent pressure-reducing valve and a solenoid valve for cavity saturation control.
Control system
The standard control system adopts PLC and HMI control mode, adopts a standard modular control board, and the control system design has been fully verified and is practical for application, stable, and reliable.
Purification filtration system
Both the supply air and exhaust an H14 HEPA filter filters air of the cavity, and the process pipeline and cavity are designed for purifying. With the high-efficiency filtration system, A-level purification can be achieved in the cavity.
EHS requirements
Personnel safety protection and product safety protection are fully considered in the design, the use of strong electric safety protection to ensure that the operator is not exposed to strong electricity, both sides of the emergency stop button design; High temperature components shall be clearly marked with high temperature; The chamber adopts electromagnetic interlock device to avoid contamination of products and clean rooms; Abnormal state audible and visual alarm signal prompts to avoid security incidents.

Design Features
1. Employing low-temperature environmentally friendly sterilization technology.
2. The sterilization process is fast and cost-effective.
3. The efficiency of the high-efficiency filter is H14 (EN1822), which can provide laminar airflow protection and reduce cross-contamination in the inner/outer areas.
4. Internal chamber made of stainless steel with rounded corners, which is corrosion-resistant and easy to clean.
5. Equipping mechanical or electronic interlocking system to prevent cross-contamination of airflow.
6. The noise level is less than 60 dBA during operation.
7. Seal strips are installed on both door frames to ensure air tightness.
8. Unique arc door design, beautiful and easy to clean (optional).
9. Reinforced the structure design for easy installation and compatibility with cleanrooms.
PRODUCT SPECIFICATIONS
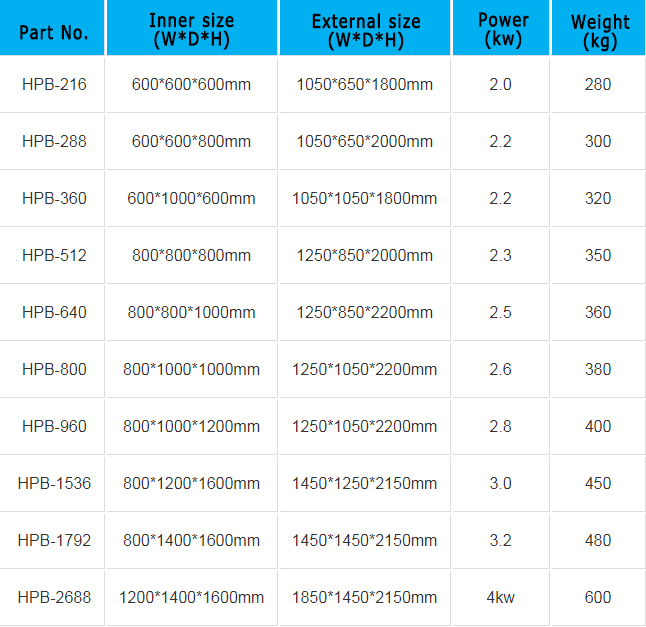
Note: The above are standard products, non-standard products can consult our sales staff, we will provide non-standard customized products according to customers’ requirements.
STERILIZATION PROCESS
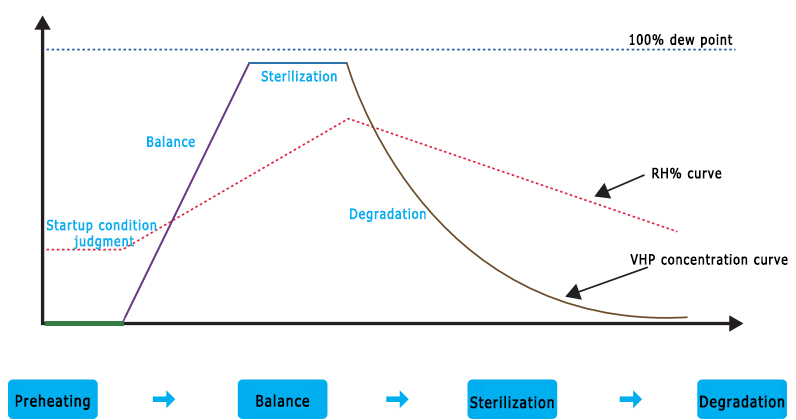
Preheating: The temperature and humidity conditions of the chamber are automatically adjusted before the equipment is started to achieve the set program start conditions.
Balance: The sterilization conditions are started, and the device performs self-balancing of VHP concentration and saturation to achieve sterilization conditions.
Sterilization: Start sterilization and accumulate the LOG value of sterilization until the end of sterilization.
Degradation: After sterilization, the equipment enters the exhaust degradation stage, and VHP is discharged and degraded until the end of the procedure.





